Infectious Diseases: An Overview
Understanding how infectious diseases impact the immune system and vice versa is a key piece of BRI’s work. Our team aims to better understand how germs interact with the immune system and examine if and how vaccines and medicines help fight infectious diseases. Our research spans many infectious diseases, ranging from influenza to Ebola. Our team also made crucial contributions to COVID-19 research during the pandemic, from exploring important questions about the virus to helping test the first COVID-19 vaccines.
What Are Infectious Diseases?
Infectious diseases are illnesses caused by invaders such as viruses, fungi and bacteria. These harmful germs cause infections, which happen when germs make copies of themselves and increase in number. Infections cause different symptoms depending on where they are in the body, ranging from fever and chills to stomach pain and nausea and many other symptoms. Some of the most common infectious diseases include:
- The common cold
- The flu (influenza)
- Stomach flu (gastroenteritis)
- COVID-19
What Is the Latest Research Into Infectious Diseases?
BRI’s infectious disease research includes:
- Examining how infectious diseases impact the human immune system and vice versa.
- Understanding how vaccines work in people with immune system diseases.
- Understanding if and how infections trigger autoimmune diseases.
Labs Studying Infectious Disease
Hamerman Lab
The lab is interested in understanding how myeloid cells contribute to both productive and pathological immune responses during infection, inflammation, and autoimmune diseases.
Lacy-Hulbert Lab
Researchers in the Lacy-Hulbert Lab study how components of the immune system work together to defend against harmful invaders while avoiding damage to healthy tissues or harmless microbes.
Stefani Lab
The goal of our research is to better understand mechanisms of sensing and repair during diseases like cancer, infections, and neurodegenerative disorders.
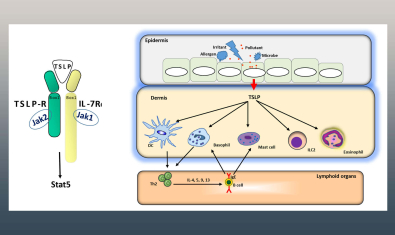
Ziegler Lab
The Ziegler laboratory is investigating the role of the epithelial cytokines TSLP and IL-33 in regulating protective responses at mucosal barrier surfaces such as the respiratory and gastrointestinal tracts.
Infectious Disease Clinical Research
Studies labeled as “Enrolling” are actively recruiting new participants while studies labeled as “Closed to Enrollment” are still active but no longer seeking new participants.
Please email the Clinical Trials Unit or call (206) 287-6260 for more information.
- Multiple Patient Program for Lamprene® (clofazimine) for the treatment of Non-Tuberculous Mycobacterial (NTM) Infections
Learn more at Clinicaltrials.gov
Principal Investigator: Kathleen Horan, MD
Status: Enrolling
- A PHASE 3 MASTER PROTOCOL TO EVALUATE ADDITIONAL DOSE(S) OF BNT162b2 IN HEALTHY INDIVIDUALS PREVIOUSLY VACCINATED WITH BNT162b2
Learn more at Clinicaltrials.gov
Principal Investigator: Uma Malhotra, MD
Status: Closed to Enrollment