Our laboratory is focused on the factors that control immune responses, both normal and pathogenic, at mucosal barrier surfaces. These surfaces in the respiratory and gastrointestinal tracts protect from external insults such as virus and bacterial infections. However, they also can respond inappropriately to innocuous stimuli such as allergens and food.
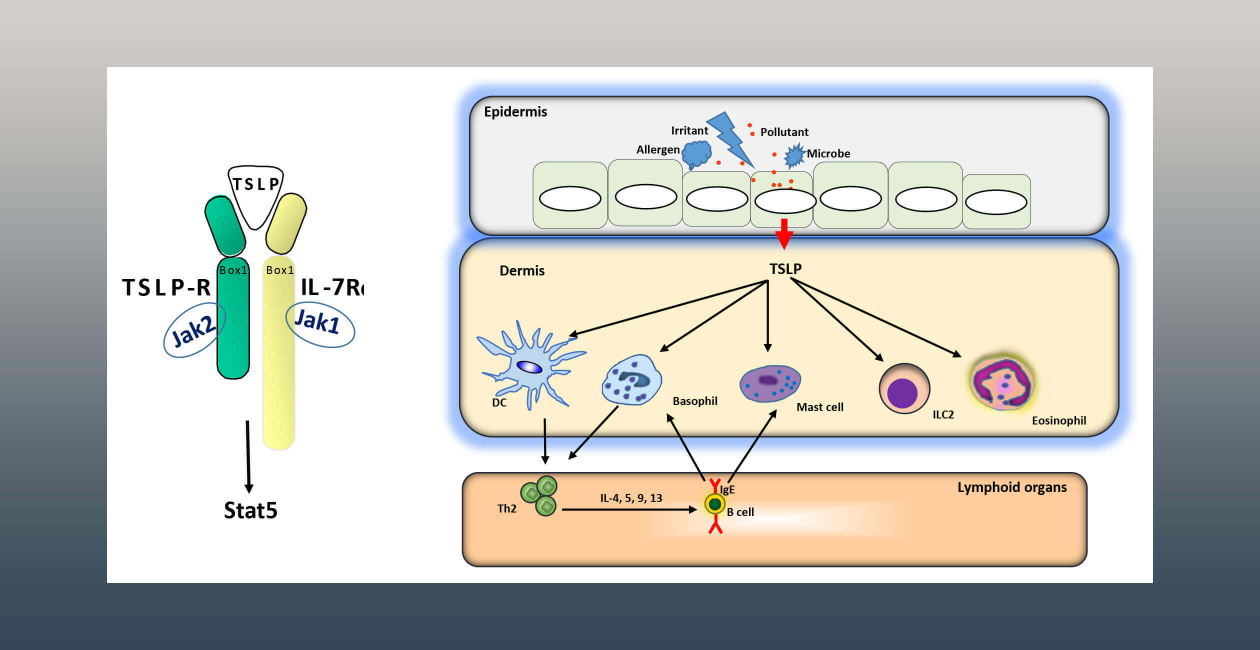
The laboratory is investigating the role of the epithelial cytokines TSLP and IL-33 in regulating responses at these barrier tissues. These cytokines, which are referred to as “alarmins,” are released rapidly by epithelial cells following infection. They work to coordinate the innate and adaptive immune responses to mitigate the perceived threat.
We have also found that these cytokines, especially TSLP, are involved in cancers of the epithelium. TSLP serves as a survival factor for tumors, and also acts to blunt the immune response to the cancer.
The lab is also interested in the transcription factor Foxp3 and its role in the development and function of regulatory T cells (Tregs). These studies address the role of Foxp3 isoforms on Treg function, using both human and mouse model systems. We have also recently discovered a novel mechanism of Treg-mediated suppression-inhibition of translation. We are pursuing these studies to better understand the mechanisms that underlie Treg dysfunction in settings of autoimmunity.

Steven Ziegler, PhD
Lab Members

Finn Barry

Zoe Bishop

Honyin Chiu, PhD

Alison Greenlaw, PhD

Isaiah Melgar

Ruth Penn

Florence Roan, PhD

Tomoaki Takao, PhD

Stephanie Varela

Kristin Weinstein, BS
Research Projects
Regulation of Humoral Immunity by Thymic Stromal Lymphopoietin
Cell-intrinic regulation of germinal centers by Thymic Stromal Lymphopoetin Receptor (TSLPR) signaling
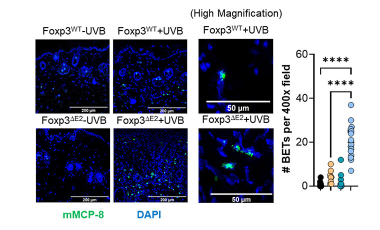
Study of Foxp3ΔExon2-expressing regulatory T cells in humans and mice
Characterization of IgE-mediated photosensitivity in Foxp3ΔExon2-expressing mice
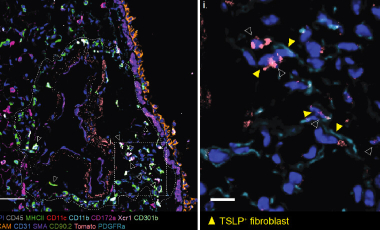
The role of TSLP and IL-33 in tissue homeostasis and inflammation
The Ziegler lab is investigating how TLSP and IL-33 signaling at adventitial sites in the lungs and skin maintain tissue immune homeostasis and regulate inflammation and tissue repair.
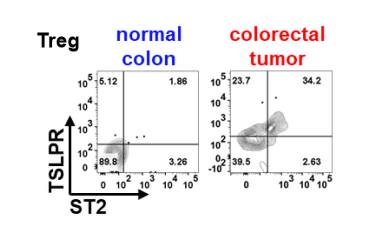
The Role of TSLPR+St2+ Tregs in colorectal cancer
Diagnosis and therapy for colorectal cancer targeting a novel subset of Tregs.