That closer look revealed exactly how this altered version was different from the regular version — and something even more significant: People without immune system diseases have about equal amounts of both the regular and altered versions of the protein. But in people with lupus, the altered version outnumbers the regular version by about 4 to 1.
Now, Dr. Ziegler has earned an NIH grant to learn more about the role this protein plays in lupus.
“Our goal is to understand all of the players — the cells, the processes, the mechanisms — involved in lupus,” Dr. Ziegler says. “Then we can target those players to stop the disease.”
Using Lab Models To Get to the Root of Lupus
Lupus is a complex and little-understood autoimmune disease. Symptoms can be anything from a rash, to joint pain to kidney problems. These symptoms may come and go or mimic other diseases. Many people wait years for a diagnosis that explains their symptoms and even longer for an effective treatment.
That’s why better diagnostics and treatments are so important.
Dr. Ziegler and his team started by building a lab model to study the altered protein and the role it plays in lupus.
“In a lab model, you manipulate individual cells or proteins and see the effects in a way that you can’t in people,” Dr. Ziegler says.
The first model had extra copies of the altered protein, which led to “subclinical” symptoms of lupus like signs of underlying inflammation and kidney problems. Then the lab team added a common trigger for a lupus flare: UV light. That led to a massive inflammatory response.
The team also found that the models were producing way too much of an antibody called IgE. This could mean that a problem with the germinal center, which makes antibodies, could help explain why lupus happens.
From Lab Models to Human Samples
The second piece of this grant is in collaboration with Judith James, MD, PhD, of Oklahoma Medical Research Foundation (OMRF). Dr. Ziegler and Dr. James will examine blood samples from people with lupus and a related autoimmune disease called mixed connective tissue disease.
“At BRI, we often work in an iterative process of going between lab models and human samples,” Dr. Ziegler says. “The lab models allow us to ask questions, change variables and unravel the processes in a way that we couldn’t in people. But the human samples are crucial to helping us ask the questions that are most relevant to people with the disease.”
Between samples from the biorepositories at BRI and OMRF, the research team will examine thousands of samples and ask the following questions:
- Which forms of the proteins do the donors have?
- How much of each form?
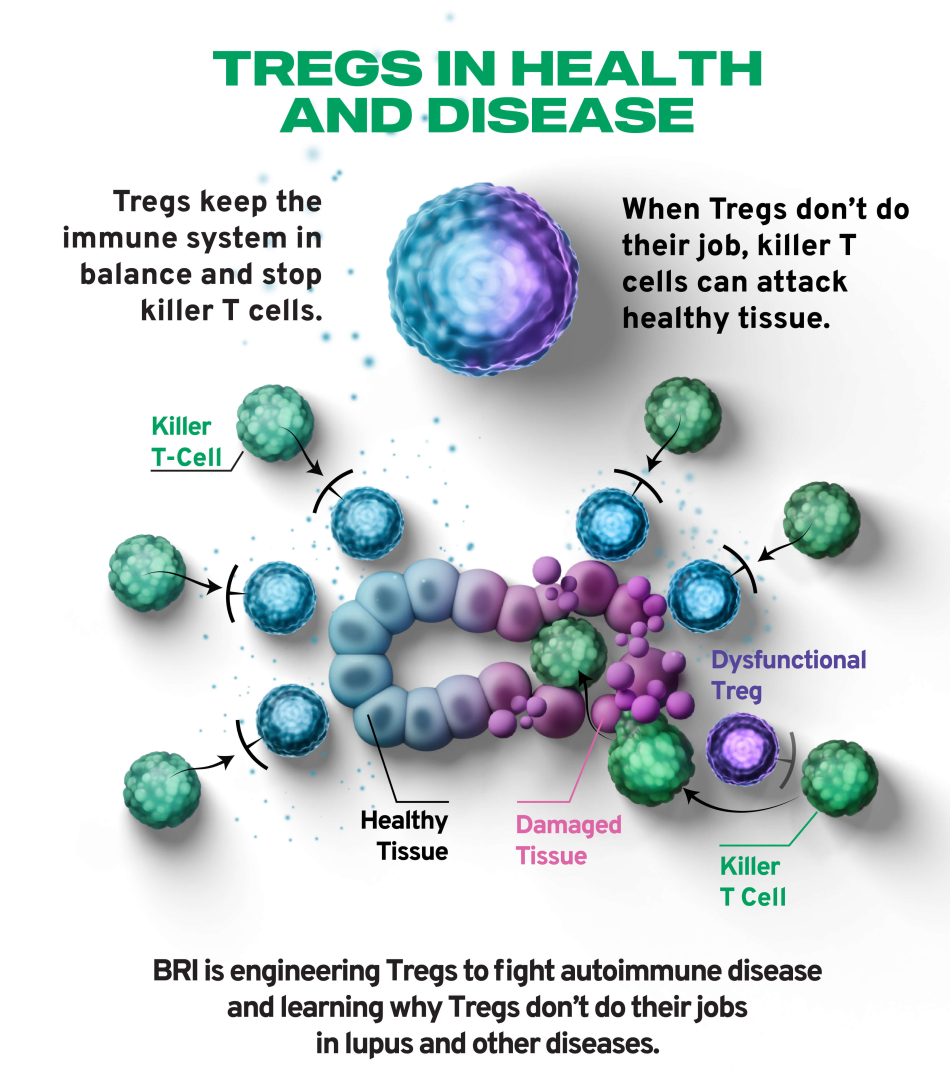
- How do those proteins affect Treg function?
- How much IgE do they have?
These answers could pave the way for better, more targeted treatments.
“We want to know, down at a very fundamental level, down to individual cells, what’s going wrong in lupus,” Dr. Ziegler says. “Figuring that out will give us a target. And once we have a target, we can design a drug or antibody or whatever you need to hit that target. And that’s how progress is made.”