“For the first time, BRI researchers have identified and are able to target a unique type of cell that causes allergies. Up until now, we couldn’t easily identify the ‘bad guy’ cells triggering allergies from the ‘good guy’ cells protecting the body,” says Steven Ziegler, PhD, who leads BRI’s immunology research program. “This makes allergy research much more straightforward and opens the door to therapies that could target this common enemy and transform treatment.”
Allergies affect tens of millions of people in the United States – including several million children – and food allergies have become more prevalent in recent years. Allergies are caused when the immune system detects an allergen such as pollen, peanuts or pet dander, and overreacts by producing antibodies to fight the allergens. The antibodies release histamine and other chemicals that cause anaphylaxis, sneezing and other symptoms.
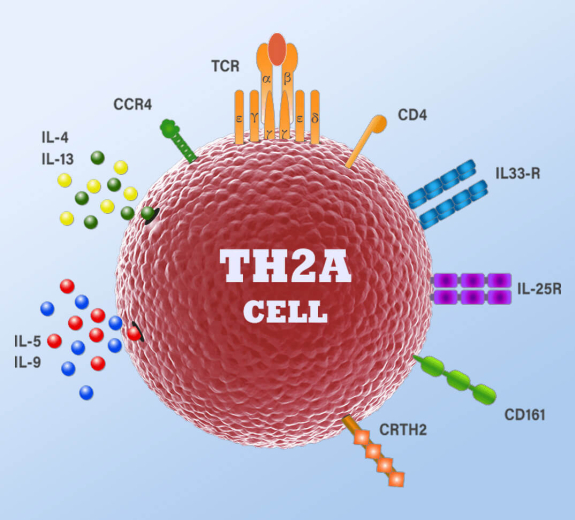
BRI’s study, led by Erik Wambre, PhD, began seven years ago, by examining a type of immune cell, called a Th2 cell, that helps orchestrate how the immune system responds to parasites, viruses, bacterial infections but also leads to allergies.
As Wambre and his colleagues analyzed blood samples containing these cells, they discovered a specialized subtype of cell, which they called Th2A, which is present in people with allergies but almost entirely absent from people who don’t have allergies. The researchers found that, unlike normal Th2 cells, these Th2A cells lack expression of one key protein – called CD27 – while simultaneously expressing the CRTH2 and CD161 proteins – that lead the immune system to overreact to allergens.
“This indicates that the Th2A cells are more specialized than conventional Th2 cells – they’re tailored to help the body respond to allergens,” Wambre says.
Wambre and his colleagues performed tests to confirm that Th2A cells play a pivotal role in at least six common allergies – including peanut, grass pollen, mold, cat dander, tree pollen and dust mites.
For instance, the researchers examined Th2A cells in blood samples from people allergic to grass pollen. They found that the Th2A cells were activated during allergy season, when they expressed larger amounts of CD38 – a protein that reflects activation following allergen exposure. CD38 was not expressed in conventional Th2 cells or outside of allergy season.
The research team also analyzed Th2A cells in blood samples from participants in a clinical trial of a new therapy for peanut allergies. Wambre and his colleagues found that the Th2A cells were activated when participants were exposed to the peanut allergen. The researchers also showed that the number of Th2A cells decreased as participants became more tolerant of the peanut allergen.
“This is the first time we’ve had a way to accurately measure the allergy process and assess whether therapies are working,” Wambre says.
Most important, researchers can now pursue therapies that potentially disarm Th2A cells and stop allergies.
The study’s results have already caught the attention of leading allergy research and advocacy organizations including Food Allergy Research & Education (FARE), which awarded Wambre a five-year Mid-Career Investigator Award in 2015.
“This could make allergy research much more directed, since scientists can now focus on the specific cells involved in generating allergies,” says James R. Baker, Jr., CEO and Chief Medical Officer of FARE, which funds research on new allergy therapies. “We’re hopeful that studying Th2A cells will quickly improve our understanding of how allergies develop, and lead to therapeutic approaches to block allergies. This would improve the lives of allergy sufferers tremendously.”