
A Decades-Long Dream Becomes Reality
The First Trials of Treg-Based Therapies
For Jane Buckner, MD, the discovery of Tregs in the late 1990s sparked an idea for better, more targeted autoimmune disease therapies.
Many autoimmune disease treatments slow down the entire immune system, not just the cells that cause disease. This leaves people vulnerable to infections and cancer. Dr. Buckner especially noticed how Tregs have the power to restrain the immune system in a very specific way: They pump the brakes on only the cells that attack healthy tissue.
“Developing a Treg-based therapy to treat autoimmune diseases has been a goal for most of my career,” Dr. Buckner said. “And the first clinical trials of this type of therapy for type 1 diabetes (T1D) just began.”
Laying the Groundwork
The first step in using Tregs as a therapy was figuring out exactly where these cells go wrong in autoimmune disease. Research from the Buckner Lab revealed that the problem wasn’t with the Tregs themselves. It was that in people with autoimmune disease, their attacker cells didn’t respond when Tregs told them to stop attacking.
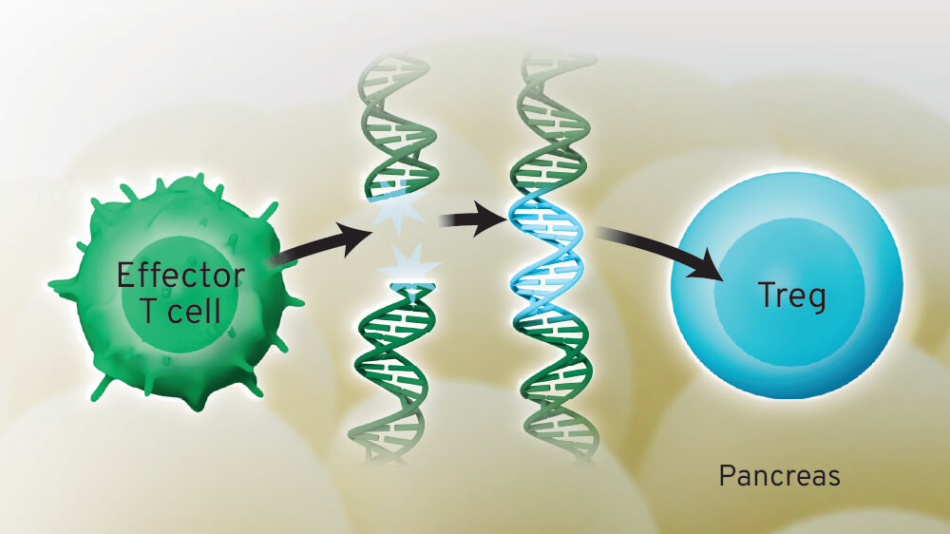
That finding led to a series of questions about how to create Tregs that would curb the attack. The next 20 years of research revealed answers that moved the possibility of a Treg-based therapy closer to reality. Building on a revolutionary approach that reprograms a person’s T cells to attack cancer, Dr. Buckner’s vision was to do the opposite in autoimmune diseases: Turn attacker cells into Tregs, so they stop attacking.
In partnership with David Rawlings, MD, at Seattle Children’s, the research team used gene editing to turn attacker T cells in T1D into Tregs. Then came a series of tests using samples from people with T1D, which revealed that engineered Tregs could in fact curb the cells that cause T1D in the lab. These tests paved the way to use this approach in people.
BRI's Mission in Action
January 2026 marked a huge milestone: The first Treg-based therapy for T1D entered clinical trials in people. The trial will ask two crucial questions: Is it safe and does it work? If so, Dr. Buckner and her team will examine how long these treatments work for and how to make them widely accessible.
“My hope is that we can rapidly invest in making this drug relatively inexpensive, easier to administer and available to everyone who needs it,” Dr. Buckner said.
Harnessing the power of Tregs doesn’t stop with T1D: Dr. Buckner’s team has already developed Tregs that could target the joints in rheumatoid arthritis and the liver in a rare autoimmune disease called primary biliary cholangitis. This approach holds the promise to work for many other autoimmune diseases and allergies too.
“We started by understanding the fundamental biology and built on that knowledge to inform better care,” Dr. Buckner said. “This is what BRI exists to do and it’s a significant step toward our vision of a healthy immune system for everyone.”
Immuno-what? Hear the latest from BRI
Keep up to date on our latest research, new clinical trials and exciting publications.


