The Khor Lab is pioneering new ways to understand how the immune system works and why it sometimes goes awry. Using advanced tools to study antigen-specific immune cells, the lab seeks to uncover how immune responses are dysregulated in people with Down syndrome. This work sheds light on challenges unique to this population while also revealing broader insights into aging, autoimmunity and vaccine responses.
A central focus of the Khor Lab’s research is discovering novel pathways that regulate immune tolerance and inflammation, with the goal of informing precision therapies tailored to individual patients. By studying the human immune system in depth, researchers are identifying promising new targets for treatment and uncovering shared principles that connect seemingly distinct immune system diseases.
Collaboration is fundamental to the lab’s approach, bringing together experts in genetics, chemistry, computation and clinical research. Trainees are supported in a creative, rigorous and collaborative environment, where innovative approaches to clinically relevant questions are encouraged.

Bernard Khor, MD, PhD
Lab Members

Emma Bols

Suzie Ruskin
Research Projects
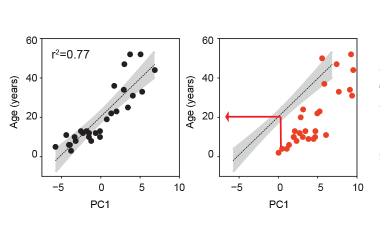
Aging and Down Syndrome: The Immune System and Beyond
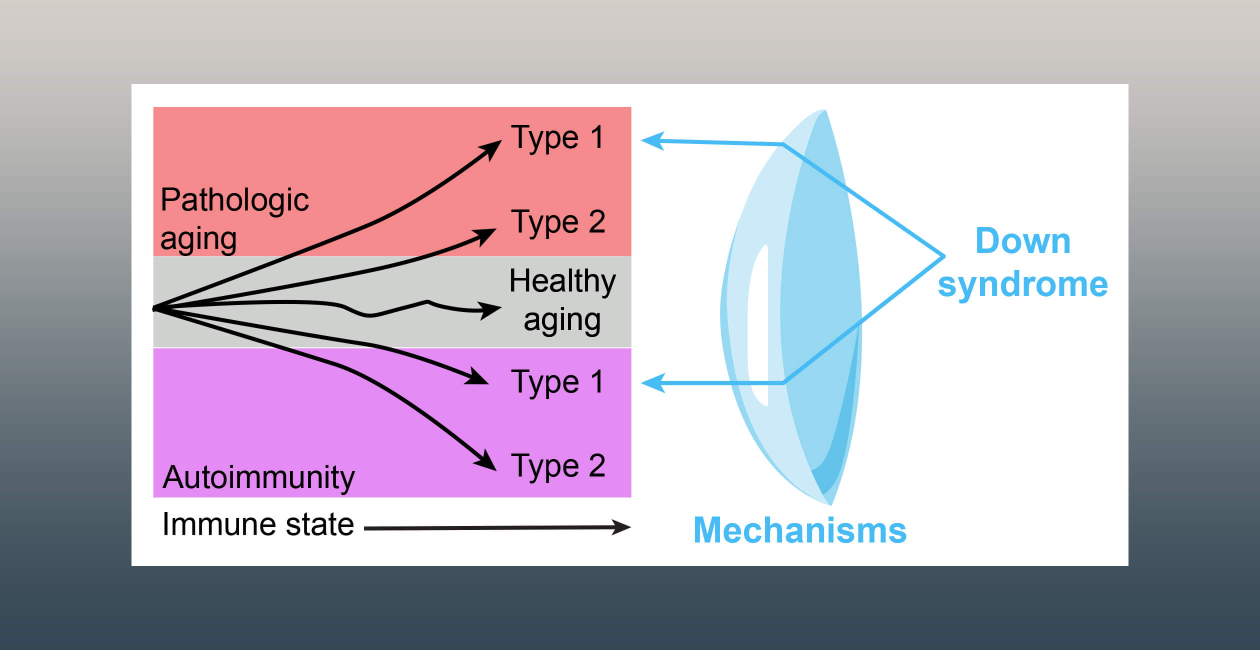
People with Down syndrome often experience health challenges that resemble aspects of accelerated aging, including a higher risk of infections and reduced protection from some vaccines, such as influenza and COVID-19.
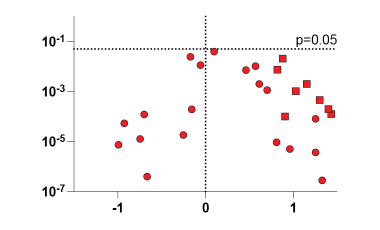
Dissecting the Mechanisms Driving Autoimmunity in People With Down Syndrome
People with Down syndrome face a strikingly higher risk of developing autoimmune disease — in some cases up to 100 times greater than in the general population.
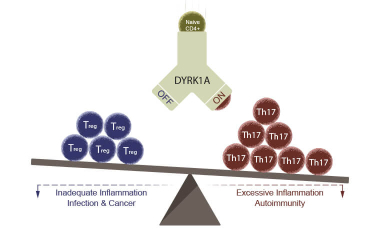
Understanding How DYRK1A Regulates T Cell Biology
A healthy immune system depends on balance. Inflammation is essential for fighting infection, but when it becomes excessive or misdirected, it can drive autoimmune and inflammatory disease.