Introduction
The Buckner Lab is focused on identifying the underlying mechanisms by which regulation of the adaptive immune response fails or is overcome in the setting of human autoimmunity. Our multipronged approach integrates the study of genes associated with disease risk, the study of lymphocyte populations implicated in the pathogenesis of autoimmune disease and the analysis of carefully curated biological samples from well-characterized subjects with type 1 diabetes, rheumatoid arthritis, multiple sclerosis, systemic lupus erythematosus and relapsing polychondritis.
The goal in all of our studies is to understand how pathways known to regulate the immune response are altered in the setting of disease, and further when and how these mechanisms of failed tolerance contribute to the development and progression of human disease.

Jane Buckner, MD
Lab Members

Aleah DeSchmidt

Victor Lui, PhD

Ethan McClain

Arpit Mishra, PhD

Emma Mortensen

Sylvia Posso

Cliff Rims

Jing Song, PhD

Megan Tatum, BS

Ritika Tewari, PhD
Soo Jung Yang, PhD
Research Projects
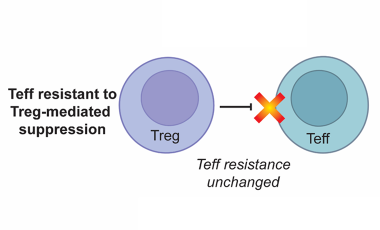
Altered T cell function in human autoimmune disease
The Buckner lab has a long-standing interest in understanding how T cell function is altered in human autoimmune disease.
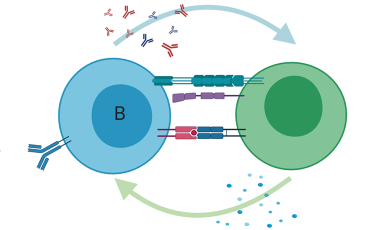
Antigen-specific T cells in autoimmunity
Antigen-specific T cells play a central role in the adaptive immune response, driving the cellular response to foreign antigens and maintaining immune tolerance to self-antigens as well as contributing to the formation of immunological memory.
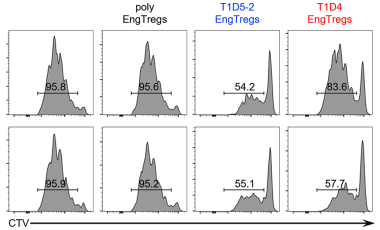
Regulatory T cells in the context of autoimmune disease
Regulatory T cells (Tregs) defined by expression of CD4, CD25 and the transcription factor FOXP3 play an essential role in maintaining immune tolerance and preventing the development of autoimmunity by suppressing effector T cell (Teff) proliferation and cytokine production.
Featured Publications
-
IL-6-targeted therapies directed to cytokine or receptor blockade drive distinct alterations in T cell function.
JCI InsightSpeake C, Habib T, Lambert K, Hundhausen C, Lord S, Dufort MJ, Skinner SO, Hu A, Kinsman M, Jones BE, Maerz MD, Tatum M, Hocking AM, Nepom GT, Greenbaum CJ, Buckner JH -
Pancreatic islet-specific engineered Tregs exhibit robust antigen-specific and bystander immune suppression in type 1 diabetes models.
Sci Transl MedYang SJ, Singh AK, Drow T, Tappen T, Honaker Y, Barahmand-Pour-Whitman F, Linsley PS, Cerosaletti K, Mauk K, Xiang Y, Smith J, Mortensen E, Cook PJ, Sommer K, Khan I, Liggitt D, Rawlings DJ, Buckner JH -
IL-6-Driven pSTAT1 Response Is Linked to T Cell Features Implicated in Early Immune Dysregulation.
Front ImmunolLambert K, Diggins KE, Jones BE, Hundhausen C, Maerz MD, Hocking AM, Sanda S, Greenbaum CJ, Linsley PS, Cerosaletti K, Buckner JH -
Fewer LAG-3+ T Cells in Relapsing-Remitting Multiple Sclerosis and Type 1 Diabetes.
J ImmunolJones BE, Maerz MD, Bahnson HT, Somasundaram A, McCarthy LH, Speake C, Buckner JH -
Shared recognition of citrullinated tenascin-C peptides by T and B cells in rheumatoid arthritis.
JCI InsightSong J, Schwenzer A, Wong A, Turcinov S, Rims C, Martinez LR, Arribas-Layton D, Gerstner C, Muir VS, Midwood KS, Malmström V, James EA, Buckner JH