Consistency is paramount for high quality data leading to novel scientific discoveries. This is of particular importance for longitudinal studies, clinical trials, or when samples that are intended to be compared are assayed far apart in time.
The HIP core employs various approaches to ensure data comparability over time. We offer dedicated hands to maintain consistency within and across studies. By centralizing efforts and instrumentation, we streamline the process and minimize variation. We adhere to standard operating procedures (SOPs) and keep up with the best practices in the field.
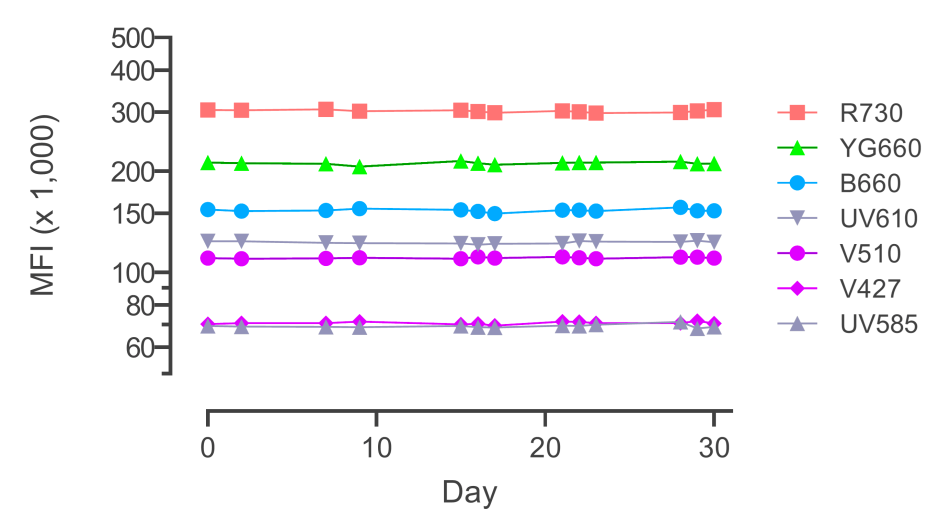
To minimize technical variation at different stages of a study, we have implemented several techniques. For example, we have investigated whole blood preservation reagents and assessed the impact of delays and different storage conditions on the quality of peripheral blood mononuclear cell (PBMC) isolation. During sample processing, we employ strategies such as intelligent batching, barcoding, and freezing of CyTOF panel staining cocktails. At the instrument level, we utilize beads to ensure consistent performance settings over time and incorporate internal controls to monitor expected variations.
By employing these strategies, we ensure the generation of the most consistent and high-quality data necessary for scientific discoveries.
Featured Publications
-
Guidelines for standardizing T-cell cytometry assays to link biomarkers, mechanisms, and disease outcomes in type 1 diabetes.
Eur J ImmunolYang JHM, Ward-Hartstonge KA, Perry DJ, Blanchfield JL, Posgai AL, Wiedeman AE, Diggins K, Rahman A, Tree TIM, Brusko TM, Levings MK, James EA, Kent SC, Speake C, Homann D, Long SA, Immunology of Diabetes Society T-Cell Cytometry Group. -
A simple strategy for sample annotation error detection in cytometry datasets.
Cytometry ASmithmyer ME, Wiedeman AE, Skibinski DAG, Savage AK, Acosta-Vega C, Scheiding S, Gersuk VH, O'Rourke C, Long SA, Buckner JH, Speake C
Additional Research Projects
Customizing support and developing tailored assays to meet unique research needs
Human immunology intersects with many other rapidly-evolving fields such as autoimmunity, allergy, infectious disease, transplantation, and oncology.
Leveraging high-parameter single-cell technologies to study human disease
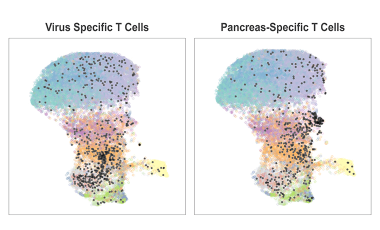
Recent advances in cytometry technologies allow us to measure an increasing number of parameters per cell, generating complex, high-dimensional single-cell datasets from limited and precious samples.


