Inflammatory Bowel Disease (IBD), consisting of Crohn’s disease and ulcerative colitis (UC), happens when the immune system lining the intestines becomes intolerant of the foreign material, most likely the bacteria, that passes through and normally fills the bowels. The Lord lab is investigating how this loss of “tolerance” happens in IBD, to learn how the immune system normally coexists peacefully in close proximity to gut contents.
While still incurable and debilitating, IBD has become much more manageable for some patients in recent years largely due to new and highly specific medications that can often stop immune cells from inflaming the intestines. By carefully examining how such medications specifically affect immune cells in the blood and intestinal biopsies of IBD patients, and correlating these changes with therapeutic success, we provide a better understanding of how IBD happens and, more importantly, how we may ultimately fix it.
Along the way, we are identifying differences in the immune systems of different IBD patients that may explain or predict disease behavior, and serve as “biomarkers” to guide a more personalized approach to treatment.

James Lord, MD, PhD
Lab Members

Ben Krautwald

Elizabeth Reznikov, DO, PhD

Donna Shows
Past Lab Members
Lord Lab
- Elisa Boden, MD — gastroenterologist and assistant professor of medicine, division of gastroenterology and hepatology, Oregon Health and Science University School of Medicine
- Duncan Hindmarch — graduate student, Oregon Health and Science University Department of Immunology
- Ramya Kongala
- Andrew Konecny — graduate student, University of Washington Department of Immunology
- Amiko Uchida, MD — gastroenterologist, University of Utah Spencer Fox Eccles School of Medicine
Translational Research Program
- Kyle Williams — graduate student, University of Colorado School of Medicine
Featured Publications
-
MAdCAM-1 Costimulates T Cells through Integrin α(4)β(7) to Cause Gene Expression Events Resembling Costimulation through CD28.
ImmunohorizonsDeBerg HA, Konecny AJ, Shows DM, Lord JD -
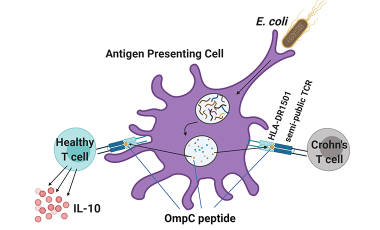
Escherichiacoli-Specific CD4+ T Cells Have Public T-Cell Receptors and Low Interleukin 10 Production in Crohn's Disease.
Cell Mol Gastroenterol HepatolUchida AM, Boden EK, James EA, Shows DM, Konecny AJ, Lord JD -
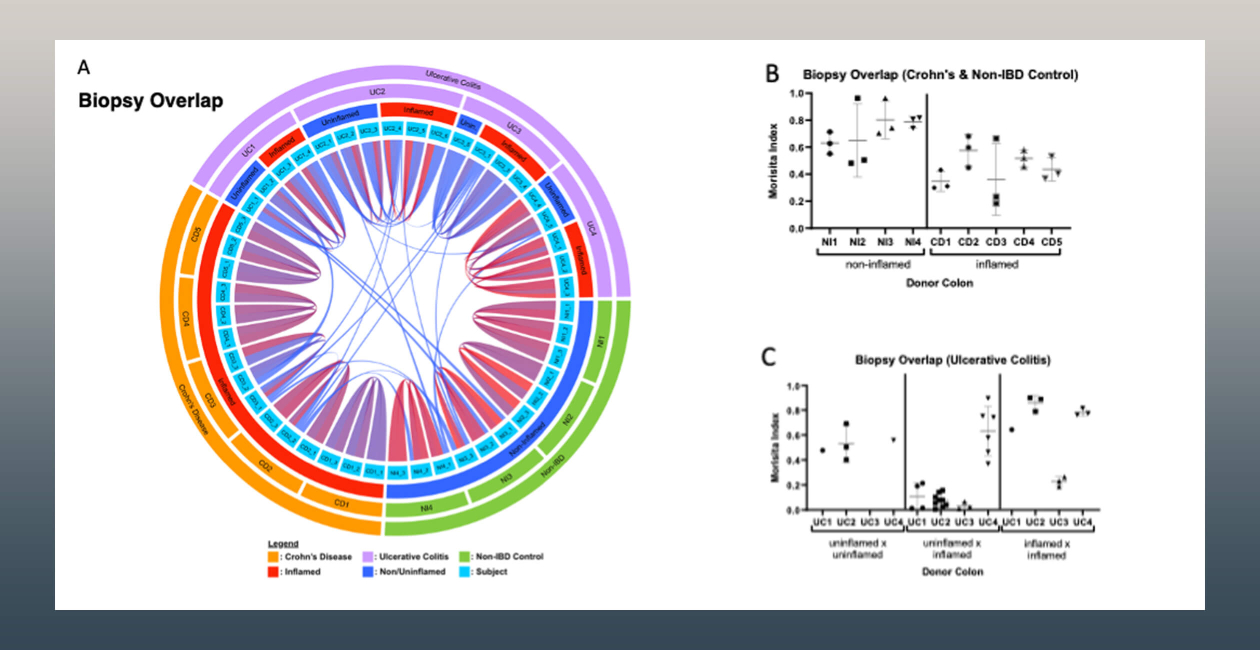
High-dimensional immune phenotyping and transcriptional analyses reveal robust recovery of viable human immune and epithelial cells from frozen gastrointestinal tissue.
Mucosal ImmunolKonnikova L, Boschetti G, Rahman A, Mitsialis V, Lord J, Richmond C, Tomov VT, Gordon W, Jelinsky S, Canavan J, Liss A, Wall S, Field M, Zhou F, Goldsmith JD, Bewtra M, Breault DT, Merad M, Snapper SB, Lord JD -
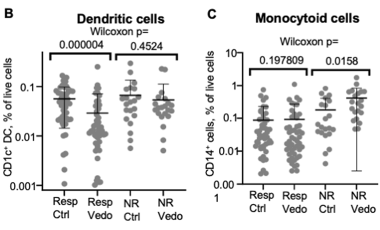
Identification of Candidate Biomarkers Associated with Response to Vedolizumab in Inflammatory Bowel Disease.
Dig Dis SciBoden EK, Shows DM, Chiorean MV, Lord JD -
Circulating integrin alpha4/beta7+ lymphocytes targeted by vedolizumab have a pro-inflammatory phenotype.
Clin ImmunolLord JD, Long SA, Shows DM, Thorpe J, Schwedhelm K, Chen J, Kita M, Buckner JH