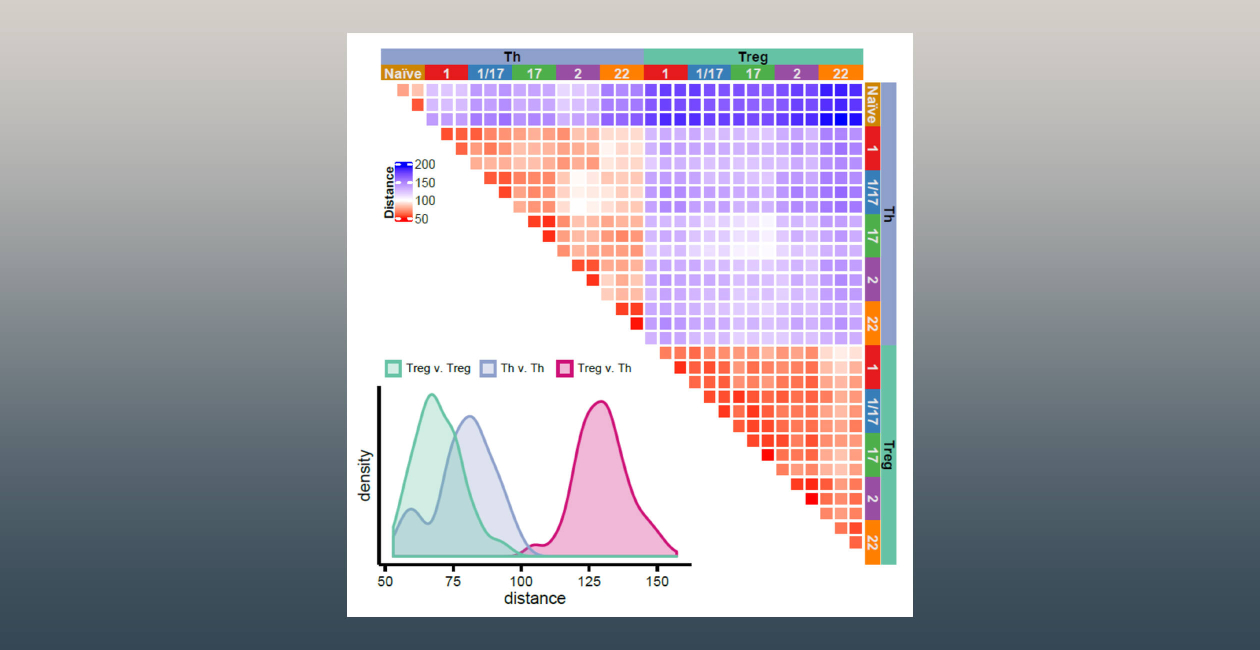
The Campbell laboratory is interested in understanding the basis for T cell activation, function and tolerance. We use both animal models and human samples to identify functionally relevant populations of effector and regulatory T cells, define the factors that promote their development and maintenance and delineate their roles in healthy immune responses and in immune-mediated diseases.
We are also work to develop and characterize new methods to promote regulatory T cell activity to treat autoimmune disease.
The lab is also studying how T cells interact with and reprogram the responses of structural cells in the skin to regulate normal tissue function, and understanding how this changes during cutaneous inflammatory diseases. We use an array of innovative techniques in this work, including transcriptional and epigenetic profiling of cells isolated from primary human skin samples, tissue explant cultures and organotypic skin culture models.

Daniel J. Campbell, PhD
Campbell Lab Members

Manreet Bhuller

Mitch Fahning

Braxton Jamison, PhD

Samuel Klebanoff

Ayako Takamori, PhD

Lauren Ziegler
Research Projects
Restoring immune tolerance via regulatory T cell-targeted IL-2 mutein therapy
Boosting regulatory T cell activity with targeted versions of IL-2 to arrest ongoing autoimmunity and restore immune tolerance.
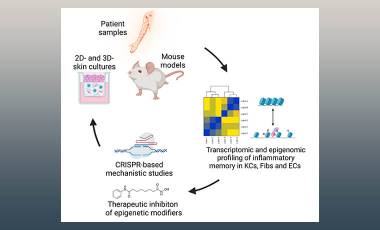
T cell-structural cell interaction in the skin
The structural cells of the skin have an important role in cutaneous immunity. This project examines how they are reprogrammed by skin-resident T cells, and how this in turn regulates inflammatory and autoimmune diseases at this site.