“The research will inform better treatments and help move us toward cures for IBD, and open the door to autoimmune discoveries that extend far beyond the gut.” Dr. Lacy-Hulbert says.
Hardwired to Respond Differently
Dr. Lacy-Hulbert thinks IBD might start deep in your gut, where your immune cells learn what — and when — to attack. In people with IBD, he thinks the immune system gets the wrong training, and learns to attack healthy tissue.
To figure out why, Dr. Lacy-Hulbert is using biorepository samples to study how people with IBD react to viruses and bacteria. He compares their reactions to those of people who don't have IBD.
“If we can understand why the immune system responds differently in IBD, we can look for ways to retrain it — which could cure the disease,” Dr. Lacy-Hulbert says.
B Cells and Gut Bacteria
Dr. Harrison started his career in England, moved to the National Institutes of Health in Maryland, and recently joined BRI to study another gut mystery: How do B cells know which bacteria the immune system should destroy?
“Everyone’s gut has millions of bacteria, but somehow the immune system knows it should only attack some of them,” he says.
If Dr. Harrison and his colleagues can learn how typical immune systems know which bacteria to attack, it could inform therapies that re-educate immune systems of people with autoimmune disease.
“We hope to learn a few tricks from balanced immune systems, so we can learn how to teach the body not to respond in IBD and other autoimmune diseases,” he says.
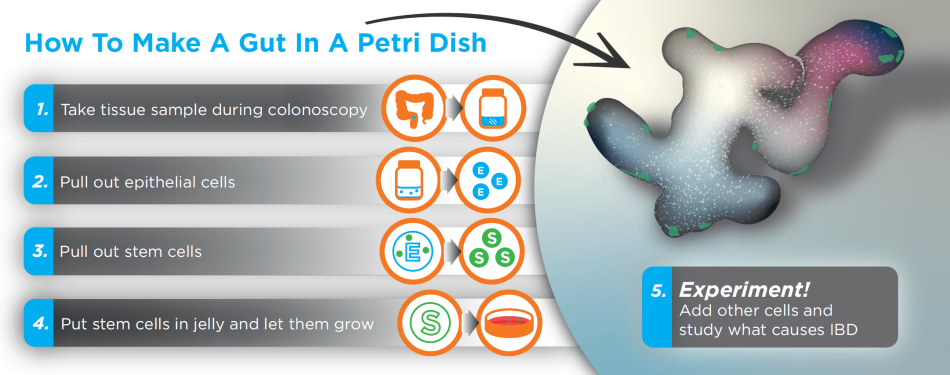
A Gut in a Petri Dish
This new program could help Dr. Lord solve a mystery that’s stumped him for nearly two decades: Regulatory T-cells are supposed to keep your body from producing too much inflammation. But people with IBD have more regulatory T-cells — and more inflammation — than people without IBD.
"It's the exact opposite of what you'd expect," Dr. Lord says.
He thinks the environment inside the gut (including bacteria, fluid and tissue) may be the problem. This program's new funding gives Dr. Lord and his colleagues the means to study this using an unprecedented tool called organoids.
“We’ll use samples from people with IBD to recreate a tiny gut in a petri dish,” Dr. Lord says. “This lets us study and manipulate the gut’s environment, which could reveal what’s wrong with these cells, so we can learn how to fix them.”
How Cures Are Made
Organoids will also help the scientists combine their expertise to accelerate progress.
“We’ve each studied our own little piece of the gut for years, but we haven’t looked much at how these systems work together,” Dr. Harrison says. “Organoids will help us do that — which could lead to a much deeper understanding of IBD and other conditions.”
This paves the way for unprecedented insight into how autoimmune conditions start, and how to stop them.
“Together, we’re more likely to understand what goes wrong in the immune system and learn how to fix it,” Dr. Lord says, “and that’s how cures are made.”
Gut Immunity and COVID-19
Even in the face of a pandemic, this team is committed to pushing their work forward. Most people have learned that COVID-19 impacts the lungs — but it can also affect the intestines. Our team is using their expertise in studying the gut to answer important questions about COVID-19.
“Virus can be detected in the intestines and in the feces — and they last a lot longer there than in the nose or throat,” Dr. Lacy-Hulbert says. “Understanding what the virus does in the intestine and how long it stays there is really important for fighting its spread.”
Dr. Lacy-Hulbert’s team has studied how cells can become resistant to viruses like Ebola and the flu, and they’re applying the same techniques to study how SARS-CoV2, the virus that causes COVID-19, infects intestine and lung cells.
“We want to be part of a solution to this pandemic, and we have the skills and the tools to do so,” Dr. Lacy-Hulbert says. “I have no doubt that learning more about how the immune system reacts to a novel virus will improve our understanding of immune response and autoimmunity.”