The Lacy-Hulbert Lab seeks to understand how the immune system maintains balance — mounting strong defenses against infection while avoiding harmful attacks on the body itself. When this balance is disrupted, it can lead to the development of autoimmune disease. By uncovering the genes and pathways that govern immune signaling, the lab aims to identify new strategies to restore immune health and improve patient care.
Using an integrated approach that combines genetics, biochemistry and cell biology with in vivo disease models, researchers investigate how immune signaling pathways function across diverse cell types and disease contexts.
A long-standing focus of the Lacy-Hulbert Lab is how immune cells cooperate to control inflammation through the cytokine transforming growth factor–beta (TGF-beta). TGF-beta is a central regulator of immune responses, helping to quiet inflammation and promote resolution after an immune challenge. However, TGF-beta is produced in an inactive form and must be precisely activated at the right time and place. Researchers in the Lacy-Hulbert Lab have shown that dendritic cells and macrophages activate TGF-beta through a specific cell surface receptor, alpha-v beta-8 integrin, enabling TGF-beta to signal to other immune cell populations, including T cells. Current work explores how this integrin is expressed and regulated, and whether selectively targeting this process could inform new therapeutic approaches for inflammatory bowel disease, multiple sclerosis and cancer.
More recently, the lab has identified a new role for genes involved in autophagy — the process by which cells recycle their own components during stress or damage — in regulating immune signaling. Certain autophagy pathways are activated when immune cells detect danger signals through toll-like receptors (TLRs). While TLRs are essential for recognizing infections, they can also be triggered by molecules from the body’s own cells, contributing to the development of autoimmunity. The lab’s work shows that autophagy-related pathways act as a critical brake on TLR signaling, helping prevent excessive or self-directed immune responses.
Ongoing studies examine how this regulatory system functions in B cells, plasmacytoid dendritic cells and macrophages, and how genetic variation in autophagy-related genes influences lupus disease risk, with the goal of informing future approaches to diagnosis, treatment and disease prevention.
Additional Research Projects
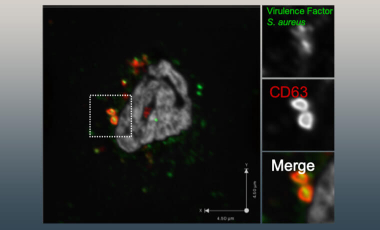
Distinguishing Pathogens From Self
Researchers in the Lacy-Hulbert Lab are exploring the molecular and cellular pathways that determine when innate immune cells promote tolerance and when they trigger inflammation.
Forward Genetics To Identify New Mechanisms in Immunity and Host Defense
In the Lacy-Hulbert Lab, researchers focus on identifying the mechanisms that control innate immunity and host defense, using innovative genetic approaches that let them explore immunity on an unprecedented scale.
SARS-CoV2 And COVID-19
During the COVID-19 pandemic, the Lacy-Hulbert Lab applied its expertise to better understand SARS-CoV-2, the virus that causes COVID-19, and explore ways to prevent and treat infection.


