Monocytes are innate immune cells that develop in the bone marrow and are continually released into circulation, where they are poised to enter tissues in response to homeostatic or inflammatory cues. Monocytes are highly plastic cells that can differentiate in tissues into a variety of monocyte-derived cells to replace resident tissue macrophages, promote inflammatory responses, or resolution of inflammation.
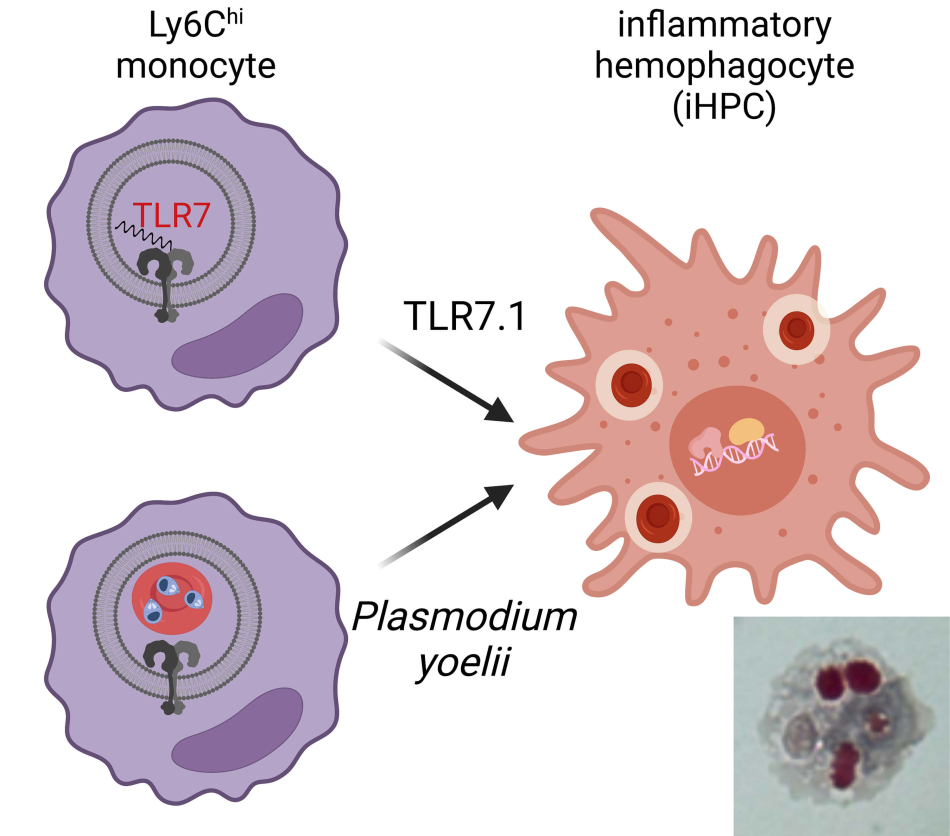
We identified a unique monocyte differentiation pathway for cells specialized for the phagocytosis of red blood cells during sustained systemic inflammation. We first identified these inflammatory hemophagocytes (iHPCs) in a mouse model of the autoimmune disease systemic lupus erythematosus (SLE) driven by transgenic overexpression of the innate endosomal RNA sensor TLR7.1. These TLR7.1 mice develop severe anemia and thrombocytopenia, reminiscent of a complication of some autoimmune diseases called Macrophage Activation Syndrome (MAS). We found iHPCs differentiated from Ly6Chi monocytes and were found in multiple blood-rich organs, including the blood, spleen, liver, and bone marrow. In TLR7.1 mice iHPC numbers correlated with anemia and thrombocytopenia in this lupus-associated MAS model, and depletion of Ly6Chi monocytes led to a rescue from MAS.
We investigated additional highly inflammatory diseases associated with anemia and we also found iHPCs differentiate in a model of severe malarial anemia caused by blood stage infection with Plasmodium yoelii where they require MyD88 and endosomal TLRs for differentiation. Therefore, during several inflammatory anemias iHPCs differentiate from monocytes and contribute to pathology.
Current projects in the lab aim to understand iHPC differentiation and function in both MAS and during severe malarial anemia. In mouse lupus-associated MAS, we are investigating signals downstream of TLR7 that promote iHPC differentiation, including the transcription factor IRF5—strongly associated with risk of lupus and other autoimmune diseases as well as important for differentiation of a variety of inflammatory macrophages.
We are also investigating human MAS associated with systemic juvenile idiopathic arthritis, the autoimmune disease where this syndrome is most frequently seen. In children with MAS, we are using single cell approaches to study circulating monocyte phenotypes, including hemophagocytes to better understand monocyte contributions to this serious disease.
Lastly, we want to understand iHPCs in severe malarial anemia using the Plasmodium yoelii 17XNL model, including their differentiation pathways, phagocytic specificities for red blood cells, and parasites, and participation in anemia. We hope our studies will lead to a better mechanistic understanding of how monocytes contribute to these diverse pathologies and help identify therapeutic targets to restrain disease.
Additional Research Projects
Flightless-1 in lung macrophage and DC development and function
Myeloid cells express a variety of proteins to regulate the actin cytoskeleton, key to migration, adhesion, and phagocytosis.
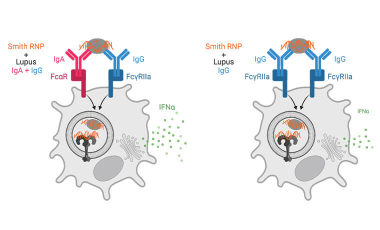
Immune complex activation of pDC IFNα production in lupus
In lupus, Plasmacytoid dendritic cells (pDCs) internalize antibody-containing immune complexes through Fc receptors thereby delivering nucleic acid containing antigens to endosomal TLRs resulting in IFNα production, which amplifies the disease.


