Peanut allergy is a significant public health problem in the United States and world-wide, with life threatening symptoms. As a result, parents have avoided feeding peanuts to children in early life, but this may prevent the development of oral tolerance to peanuts. A landmark clinical trial in 2015, the LEAP trial, showed that consuming peanuts in early childhood significantly reduced the development of peanut allergy compared with children who avoided peanuts. These results led to the new recommendation to introduce peanuts in early life and approval of peanut oral immunotherapy for individuals with peanut allergy.
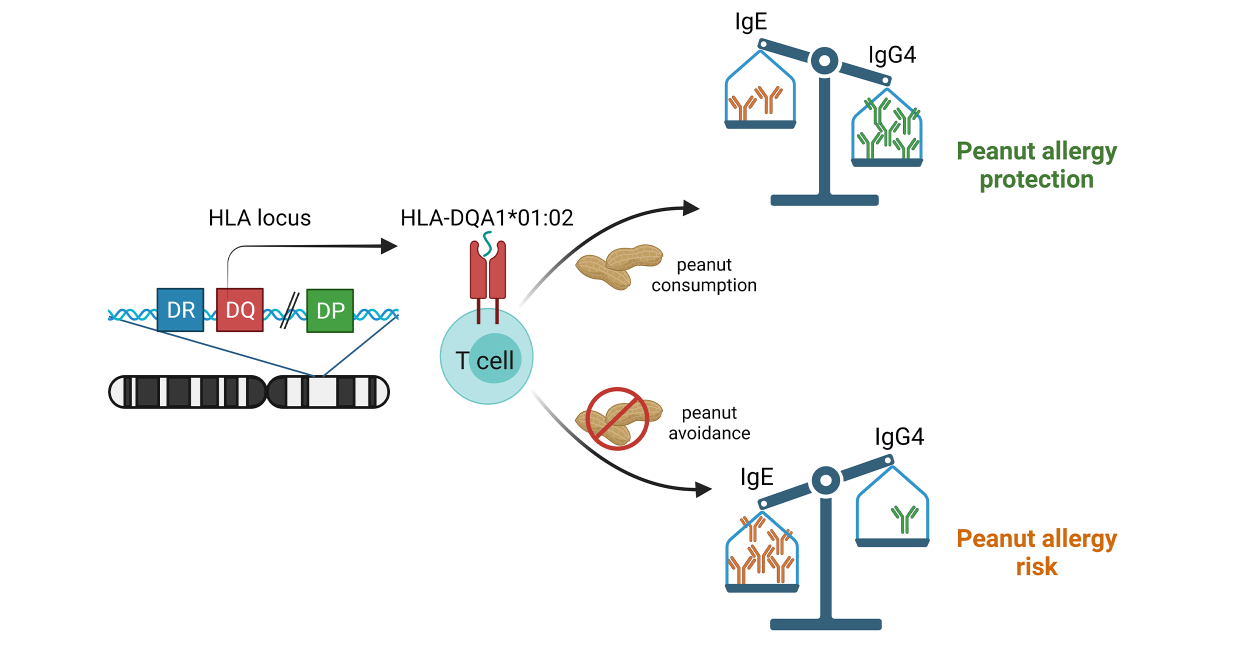
The Cerosaletti lab is interested in how genetic variation modulates allergic responses and oral immunotherapy. Consumption of peanuts results in the production of peanut specific IgG4 antibodies that are linked to protection from peanut allergy. As part of the LEAP Genetics Study Group, we showed that a specific HLA class II allele, DQA1*0102, was associated with the peanut specific IgG4 response in LEAP participants who consumed peanuts.
Remarkably, this association was driven by IgG4 specific for the Ara h2 peanut protein. Since HLA class II molecules present antigens to T cells initiating the production of antibodies, our findings suggest that HLA-DQA1*0102 presents Ara h2 peptides to T cells that support B cells to produce Ara h2 specific IgG4. To address this question, the lab is investigating the binding of Ara h2 peptides to the HLA-DQA1*0102 molecule and recognition by peanut specific T cells from allergic subjects. We are also exploring whether a genetic variant near the DQA1*0102 gene that affects its expression can further modulate this response.
Additional Research Projects
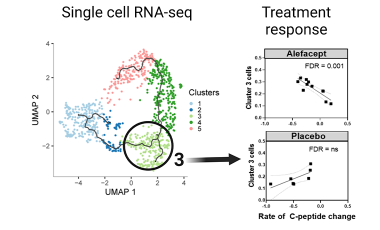
Autoreactive T cells in type 1 diabetes
The lab is investigating autoreactive effector and regulatory T cells in T1D using single cell RNA sequencing to identify features of these cells that are linked to disease course or response to therapy.
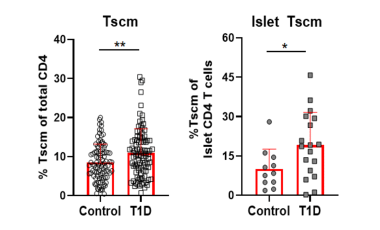
Stem-like memory CD4 T cells in type 1 diabetes
The Cerosaletti lab is studying stem-like memory CD4 T cells (Tscm) in T1D to understand the mechanism leading to expansion of these cells in T1D and determine if Tscm levels influence disease progression and response to therapy.